Alright folks, let’s dive straight into the world of chemistry and unravel one of the most fascinating molecules out there—bromine trifluoride. If you're scratching your head wondering what bromine trifluoride even is, don’t worry, you're not alone. This compound might sound intimidating at first, but by the end of this article, you'll be a pro at understanding its Lewis structure and everything that comes with it. So, buckle up and let’s get started.
Now, let’s talk about why the Lewis structure for bromine trifluoride is so important. In simple terms, the Lewis structure helps us visualize how atoms bond together in a molecule. It’s like a blueprint for understanding the chemical behavior of substances. For bromine trifluoride, this structure reveals how bromine and fluorine atoms interact, giving us insights into its stability, reactivity, and overall properties.
Whether you're a chemistry student trying to ace your exams or just someone curious about the science behind molecules, this article will break down everything you need to know about the Lewis structure for bromine trifluoride. Let’s roll up our sleeves and get to the good stuff!
Read also:Nothing Happened Zoro A Deeper Dive Into The Myth
Understanding Bromine Trifluoride and Its Significance
Bromine trifluoride, commonly written as BrF₃, is a polar interhalogen compound that plays a crucial role in various chemical processes. This molecule is highly reactive and has unique properties that make it stand out in the world of chemistry. But before we jump into the nitty-gritty of its Lewis structure, let’s take a moment to appreciate why bromine trifluoride is such a big deal.
What Makes Bromine Trifluoride Unique?
One of the coolest things about bromine trifluoride is its ability to act as both an oxidizing and fluorinating agent. This dual functionality makes it incredibly versatile in chemical reactions. Additionally, its molecular geometry and electron distribution play a significant role in determining its behavior. Understanding these aspects starts with mastering its Lewis structure.
- Bromine trifluoride is used in rocket propellants due to its high reactivity.
- It serves as a catalyst in organic synthesis.
- Its unique bonding pattern provides valuable insights into molecular behavior.
The Basics of Lewis Structures
Before we delve into the specifics of the bromine trifluoride Lewis structure, let’s quickly refresh our memory on what Lewis structures are all about. These diagrams use dots to represent valence electrons and lines to represent bonds between atoms. They provide a clear picture of how atoms share or transfer electrons to achieve stability.
Why Are Lewis Structures Important?
Lewis structures are essential tools for predicting molecular geometry, polarity, and reactivity. By analyzing the arrangement of electrons, chemists can determine how a molecule will behave in different environments. This knowledge is invaluable for designing new materials, optimizing chemical reactions, and understanding biological processes.
Breaking Down the Bromine Trifluoride Lewis Structure
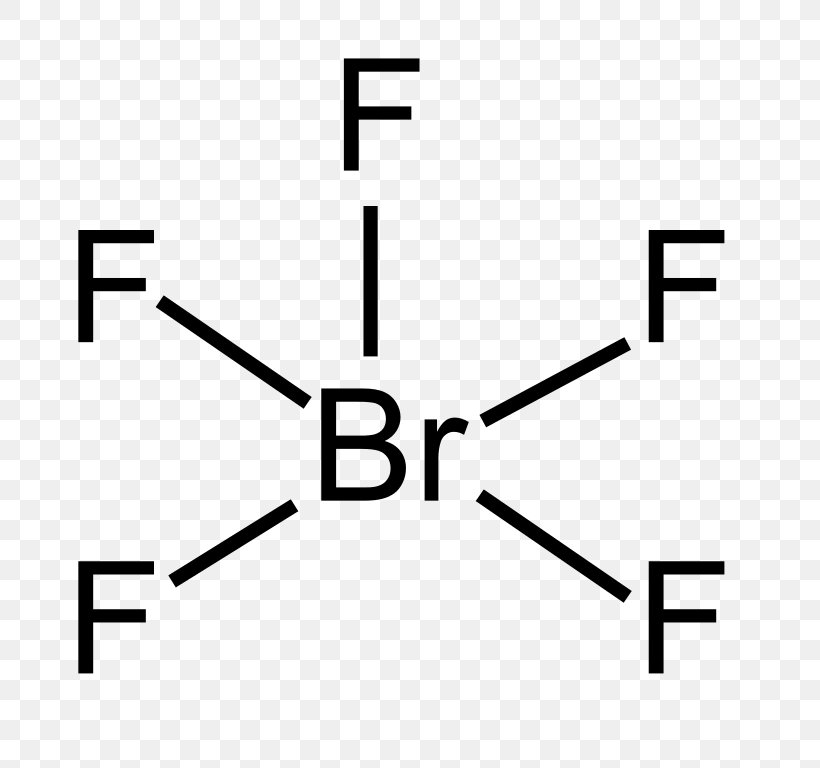
Alright, now that we’ve covered the basics, let’s get to the heart of the matter. The Lewis structure for bromine trifluoride consists of a central bromine atom surrounded by three fluorine atoms. But here’s the kicker—there’s more to it than meets the eye. Bromine also has two lone pairs of electrons, which significantly influence the molecule’s shape and properties.
Step-by-Step Guide to Drawing the Lewis Structure
Let’s walk through the process of drawing the Lewis structure for bromine trifluoride:
Read also:Victoria Ruffo The Iconic Talent Who Lit Up Mexican Television
- Calculate the total number of valence electrons. Bromine contributes 7 electrons, and each fluorine atom contributes 7 electrons, giving a total of 28 valence electrons.
- Place bromine at the center and surround it with three fluorine atoms.
- Form single bonds between bromine and each fluorine atom, using 6 electrons in total.
- Distribute the remaining 22 electrons as lone pairs on the fluorine atoms and bromine.
- Ensure that each atom satisfies the octet rule (except for bromine, which can expand its octet).
Molecular Geometry of Bromine Trifluoride
Now that we’ve drawn the Lewis structure, let’s explore how it translates into the molecular geometry of bromine trifluoride. The molecule adopts a T-shaped geometry due to the presence of two lone pairs on the central bromine atom. These lone pairs repel the bonding pairs, causing the fluorine atoms to be pushed further apart.
How Lone Pairs Affect Molecular Shape
The lone pairs on bromine play a critical role in determining the molecule’s shape. According to VSEPR theory (Valence Shell Electron Pair Repulsion), electron pairs repel each other to minimize energy. This repulsion results in the T-shaped geometry observed in bromine trifluoride.
Polarity and Chemical Properties
With its unique molecular structure, bromine trifluoride exhibits distinct polarity and chemical properties. The difference in electronegativity between bromine and fluorine creates polar bonds, making the molecule polar overall. This polarity influences its reactivity and interactions with other substances.
Applications of Bromine Trifluoride
Bromine trifluoride’s reactivity makes it highly sought after in various industries. Some of its notable applications include:
- As a powerful oxidizing agent in chemical synthesis.
- In the production of high-energy fuels for rockets.
- As a catalyst in organic reactions.
Common Misconceptions About Bromine Trifluoride
There are a few misconceptions floating around about bromine trifluoride that we need to clear up. For instance, some people believe it’s a stable compound under all conditions, which is far from the truth. Bromine trifluoride is highly reactive and can decompose violently in the presence of water or other reactive substances.
Debunking Myths
Here are a few common myths about bromine trifluoride:
- Myth: Bromine trifluoride is safe to handle. Fact: It’s highly toxic and corrosive, requiring strict safety precautions.
- Myth: It’s a nonpolar molecule. Fact: Bromine trifluoride is polar due to its asymmetrical shape.
Expert Insights and Advanced Concepts
For those who want to dive deeper into the world of bromine trifluoride, there are some advanced concepts worth exploring. From quantum chemistry to computational modeling, these tools provide a more nuanced understanding of the molecule’s behavior.
Quantum Chemistry in Action
Quantum chemistry allows scientists to calculate the electronic structure of molecules like bromine trifluoride with incredible precision. By solving complex equations, researchers can predict properties such as bond lengths, angles, and energies.
Conclusion: Unlocking the Secrets of Bromine Trifluoride
And there you have it—a comprehensive guide to the Lewis structure for bromine trifluoride. We’ve covered everything from the basics of Lewis structures to the advanced properties of this fascinating molecule. Whether you’re a student, researcher, or just a curious mind, understanding bromine trifluoride opens up a world of possibilities in chemistry.
Now, here’s where you come in. If you found this article helpful, feel free to leave a comment below or share it with your friends. Who knows? You might just inspire someone else to explore the wonders of chemistry. Until next time, keep learning and keep exploring!
Table of Contents
- Understanding Bromine Trifluoride and Its Significance
- The Basics of Lewis Structures
- Breaking Down the Bromine Trifluoride Lewis Structure
- Molecular Geometry of Bromine Trifluoride
- Polarity and Chemical Properties
- Common Misconceptions About Bromine Trifluoride
- Expert Insights and Advanced Concepts
- Conclusion: Unlocking the Secrets of Bromine Trifluoride