Ever wondered what temperature is boiling water? It's one of those questions that seems simple at first glance but gets surprisingly complex when you dig deeper. Boiling water is more than just heating it up in a pot; it's a scientific phenomenon with layers of fascinating details. So, buckle up, because we're about to uncover everything you need to know about boiling water, from the basics to the not-so-basic stuff.
Whether you're cooking pasta, brewing tea, or just curious about the science behind boiling water, this article has got you covered. We’ll explore the boiling point, factors affecting it, and some fun facts that might surprise you. Stick around, because there's more to boiling water than meets the eye!
And hey, don’t worry if you’re not a scientist. I’ll break it down in a way that’s easy to understand, with a dash of humor and real-life examples. Let’s dive in!
Read also:Win Big Play Smart Your Ultimate Guide To Ny Lottery
Here’s a quick table of contents to help you navigate:
Table of Contents:
- What is Boiling Water?
- The Boiling Point of Water
- Factors Affecting Boiling Temperature
- Why Does Water Boil?
- Boiling Water at High Altitudes
- Boiling Water in Different Containers
- Common Myths About Boiling Water
- Practical Uses of Boiling Water
- Safety Tips When Boiling Water
- Conclusion and Final Thoughts
What is Boiling Water?
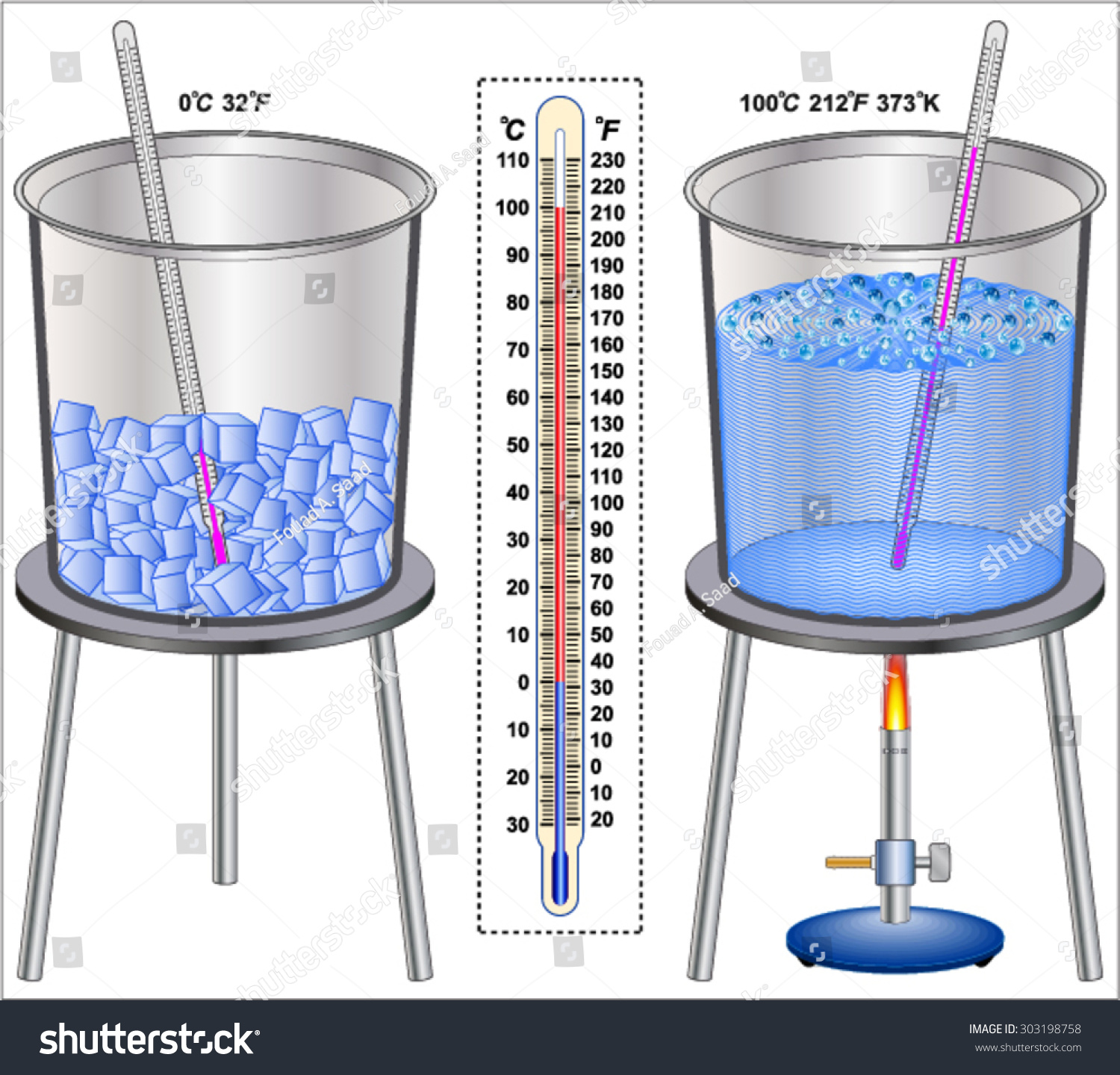
Let’s start with the basics. Boiling water is essentially the process where water reaches its boiling point and starts turning into vapor. But what exactly happens during this process? When water heats up, the molecules inside start moving faster and faster. At a certain temperature, these molecules gain enough energy to break free from the liquid state and enter the gas phase. That’s when you see those bubbles forming and rising to the surface.
Now, here’s the kicker: the boiling point isn’t always the same. It can vary depending on a few factors we’ll talk about later. But for now, let’s just say that boiling water is a fundamental part of daily life, from cooking to cleaning and even generating power.
And if you’re wondering why boiling water is so important, well, it’s not just about making your coffee taste better. Boiling water has practical applications in industries like healthcare, food production, and even space exploration. So yeah, it’s kind of a big deal.
The Boiling Point of Water
Alright, so what temperature is boiling water? Under standard atmospheric pressure, water boils at 212°F (100°C). This is the magic number most of us are familiar with. But hold on, because things aren’t always as simple as they seem.
Read also:Six Flags Santa Clarita The Ultimate Thrill Seekers Paradise You Need To Visit Now
The boiling point can change depending on the environment. For example, if you’re cooking at a high altitude, water will boil at a lower temperature. This is because the atmospheric pressure decreases as you go higher, which affects the boiling point. We’ll dive deeper into this later, but for now, just remember that 212°F is the baseline.
Why 212°F?
The boiling point of water was established based on the Celsius scale, where 0°C is the freezing point and 100°C is the boiling point at sea level. This standard was set by Anders Celsius back in the 18th century, and it’s been the go-to reference ever since.
But here’s an interesting fact: water doesn’t always boil at exactly 100°C. Impurities, like salt or sugar, can slightly increase the boiling point. So if you’re boiling saltwater, it might take a bit longer to reach that bubbly stage.
Factors Affecting Boiling Temperature
Now, let’s talk about the factors that can influence the boiling temperature of water. There are a few key players here:
- Altitude: As mentioned earlier, the higher you go, the lower the boiling point. This is because atmospheric pressure decreases with altitude.
- Impurities: Adding substances like salt or sugar to water can raise its boiling point slightly. This is known as boiling point elevation.
- Container Material: Believe it or not, the material of the pot or pan you’re using can also affect how quickly water boils. Metal containers, for instance, conduct heat better than glass or plastic.
- Heat Source: The type of stove or heating element you use can impact the time it takes for water to boil. Induction cooktops, for example, are faster than traditional gas stoves.
Understanding these factors can help you adjust your cooking methods accordingly. For instance, if you’re camping at a high altitude, you might need to cook your food longer because the water boils at a lower temperature.
Why Does Water Boil?
Boiling is a phase transition where liquid turns into gas. When water is heated, the molecules inside gain kinetic energy. At the boiling point, these molecules have enough energy to overcome the intermolecular forces holding them together in the liquid state. As a result, they escape into the gas phase, creating bubbles that rise to the surface.
But here’s the cool part: boiling is actually a form of evaporation. The difference is that evaporation happens at the surface of the liquid, while boiling occurs throughout the entire volume. This is why boiling is so much faster and more efficient for heating water.
The Science Behind It
Water molecules are held together by hydrogen bonds, which are relatively weak compared to other types of chemical bonds. When heat is applied, these bonds break, allowing the molecules to spread out and form a gas. This process requires energy, which is why boiling water takes time and effort.
And if you’ve ever noticed that boiling water makes noise, that’s because the bubbles are collapsing as they rise to the surface. It’s a fascinating process that’s both scientific and practical.
Boiling Water at High Altitudes
If you’ve ever tried cooking pasta at a high altitude, you might have noticed that it takes longer to cook. That’s because water boils at a lower temperature when you’re higher up. For every 500 feet above sea level, the boiling point of water drops by about 1°F.
For example, at an altitude of 5,000 feet, water boils at around 203°F instead of 212°F. This might not seem like a big difference, but it can significantly impact cooking times. Foods that rely on boiling water, like rice or pasta, may need to be cooked for longer periods to achieve the desired texture.
So, if you’re planning a trip to the mountains, make sure to adjust your recipes accordingly. And don’t worry, your food will still taste amazing!
Boiling Water in Different Containers
Did you know that the container you use can affect how quickly water boils? Different materials conduct heat differently, which means some pots and pans will heat up faster than others. Here’s a quick breakdown:
- Stainless Steel: A popular choice for cooking because it’s durable and heats evenly.
- Copper: Known for its excellent heat conductivity, copper pots can boil water faster than other materials.
- Aluminum: Another great conductor of heat, aluminum is lightweight and efficient for boiling water.
- Glass: While not as efficient as metal, glass containers can still be used for boiling water, especially if you’re looking for a non-reactive surface.
Choosing the right container can make a big difference in your cooking experience. If you’re in a hurry, opt for a copper or aluminum pot. But if you’re worried about chemical reactions, stainless steel or glass might be the way to go.
Common Myths About Boiling Water
There are a few myths floating around about boiling water that deserve some clarification. Let’s bust them one by one:
- Myth 1: Boiling water kills all bacteria. While boiling water does kill most bacteria, it doesn’t eliminate everything. Some spores and viruses can survive boiling temperatures, so it’s important to use additional purification methods if you’re dealing with contaminated water.
- Myth 2: Adding salt makes water boil faster. This one’s partially true. Adding salt does increase the boiling point slightly, but the difference is negligible. It’s more about flavor than speed.
- Myth 3: Cold water boils faster than hot water. This is completely false. Cold water actually takes longer to boil because it starts at a lower temperature. Always use hot water if you’re in a rush.
Now that we’ve cleared up these misconceptions, you can approach boiling water with confidence and knowledge.
Practical Uses of Boiling Water
Boiling water isn’t just for cooking. It has a wide range of practical applications in everyday life:
- Cooking: From pasta to vegetables, boiling water is essential for preparing many foods.
- Disinfection: Boiling water can be used to sterilize utensils, bottles, and other items.
- Water Purification: In areas with limited access to clean water, boiling is a simple and effective way to make water safe to drink.
- Power Generation: Boiling water is used in power plants to generate steam, which drives turbines and produces electricity.
As you can see, boiling water plays a crucial role in many aspects of modern life. It’s a versatile and essential process that we often take for granted.
Safety Tips When Boiling Water
Boiling water is generally safe, but there are a few precautions you should take to avoid accidents:
- Use a lid: Covering your pot can help water boil faster and prevent splashing.
- Handle with care: Always use oven mitts or pot holders when handling hot pots and pans.
- Keep children away: Make sure kids are supervised around boiling water to prevent burns.
- Don’t leave it unattended: Keep an eye on your pot to avoid overflow or accidents.
Following these simple tips can ensure a safe and successful boiling experience every time.
Conclusion and Final Thoughts
So, there you have it—everything you need to know about what temperature is boiling water. From the basics to the science behind it, we’ve covered it all. Remember, the boiling point of water is typically 212°F (100°C) at sea level, but it can vary depending on factors like altitude and impurities.
Boiling water is more than just a cooking technique; it’s a fundamental process with countless applications in daily life. Whether you’re making tea, sterilizing equipment, or generating power, boiling water is a versatile and essential tool.
Now that you’re armed with knowledge, why not put it to use? Try experimenting with different methods and containers to see how they affect boiling times. And don’t forget to share this article with your friends and family. Who knows, you might just inspire someone to become a boiling water expert!
Thanks for reading, and happy boiling!